DriveAFM for Polymer Chemistry
A selection of application notes by Sergei Magonov and Nanosurf
Sergei Magonov visited Nanosurf to test the DriveAFM for polymer research. He measured with CleanDrive on different polyethylene samples, on alkanes and semifluorinated alkanes and was rewarded with some quite impressive data. These data have now been collected in three application notes.
Characterization of Polyethylene with DriveAFM
Polyethylene (PE) is a semicrystalline polymer and is one of the most widely produced plastics globally for use in consumer goods and packaging with wide-ranging applications. Shopping bags, milk bottles, storage containers, toys, wires, and cables all employ polyethylene. PE is also a key component of fuel tanks and storage tanks in automobiles. The attractiveness of polyethylene lies with the combination of its physical appearance, mechanical properties such as toughness and stiffness, easy processability into various structures including films, and low manufacturing cost.

Exploring Nanoscale Organization of Normal Alkanes on HOPG Substrate with DriveAFM
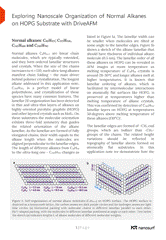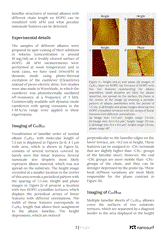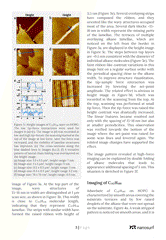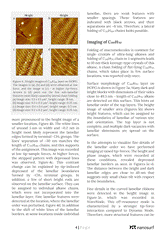
Normal alkanes: C60H122; C122H246, C242H486 and C390H782
Normal alkanes CnH2n+n are linear chain molecules, which are typically extended, and they form ordered lamellar structures and crystals. When the size of the chains increases to n > 120, such ultra-long alkanes manifest chain folding – the main driver behind polymer crystallization. The longest alkane addressed in this application note, C390H782 , is a perfect model of linear polyethylene, and crystallization of these species have many common features. The lamellar 2D organization has been detected in thin and ultra-thin layers of alkanes on highly oriented pyrolytic graphite (HOPG) and other layered crystals such as MoS2. On these substrates the molecular orientation exhibits three-fold symmetry that guides the related orientation of the alkane lamellae. As the lamellae are formed of fully elongated chains, their width equals to the alkane length when the molecules are aligned perpendicular to the lamellar edges. The length of different alkanes from C18H38 to the ultra-long one - C390H782 changes as listed in Figure 1a. The lamellar width can be smaller when molecules are tilted at some angle to the lamellar edges.
Studies of Self-Organization of Semi-Fluorinated Alkanes on Different Substrates with DriveAFM
Semifluorinated alkanes: F(CF2)n(CH2)mH
This application note concerns AFM studies of self-assembled semi-fluorinated alkanes on Silicon, HOPG and MoS2 substrates. These molecules are formed of covalently linked sequences of -CF2- and -CH2- groups with -CF3 and -CH3 terminals, respectively. Their molecular composition F(CF2)n(CH2)mH can be connoted as FnHm (Figure 1a). The compounds consist of two incompatible subunits that segregate into distinct domains like block copolymers, but without polydispersity in molecular weight. Structural ordering of such compounds is controlled by the strong incompatibility of the constituting segments, and also by the fact that the van der Waals cross-section of the flexible hydrocarbon segment is 25-30% smaller than that of the rigid helical perfluorinated segment, (Figure 1b). X-ray reflectivity studies of F14H20 films suggested that the fluorinated chains are oriented normal to the surface layer and where the alkyl segments are tilted with an angle between the two segments.
